RR Other Project Information Form
The Grants.gov RR Other Project Information form can transmit the necessary information and attachments required for your application. You should check your program announcement for instructions on how to complete the form and which attachments should be included (and the formatted associated with the attachments). Below outlines how to add the necessary attachments and other project information in Kuali Research to map to the given fields in the form.
Instructions
Once a Grants.gov opportunity containing the RR Other Project form is attached and marked as included in your federally-sponsored proposal you can complete the necessary information and Grants.gov S2S questionnaire in the Questionnaire panel to populate/map the necessary information for each section of the form.
1. Human Subjects
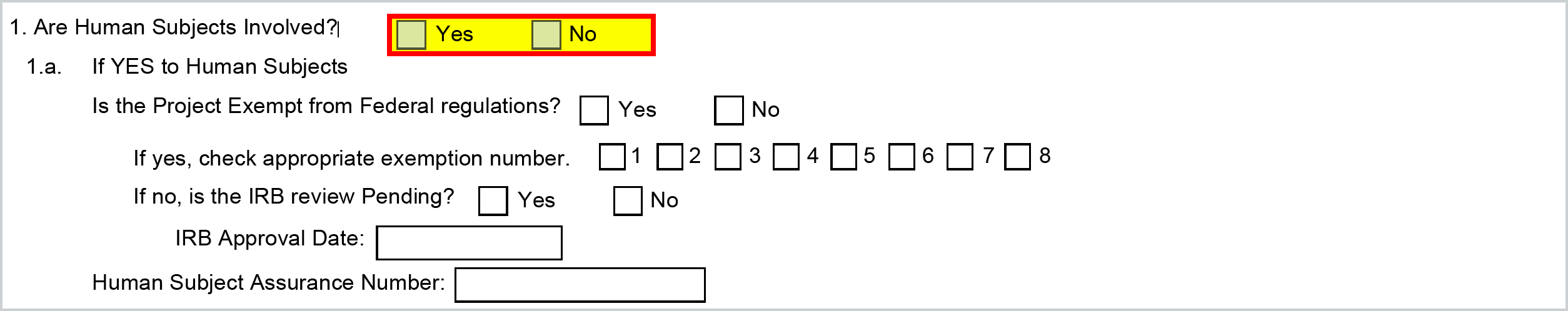
The Human Subjects information will map based on the Special Review information you have entered in the Compliance tab of the proposal in Kuali Research. If nothing is added for Human Subjects in Compliance then this will be marked NO. If a Special Review of Human Subject has been added in Compliance it will mark as YES.
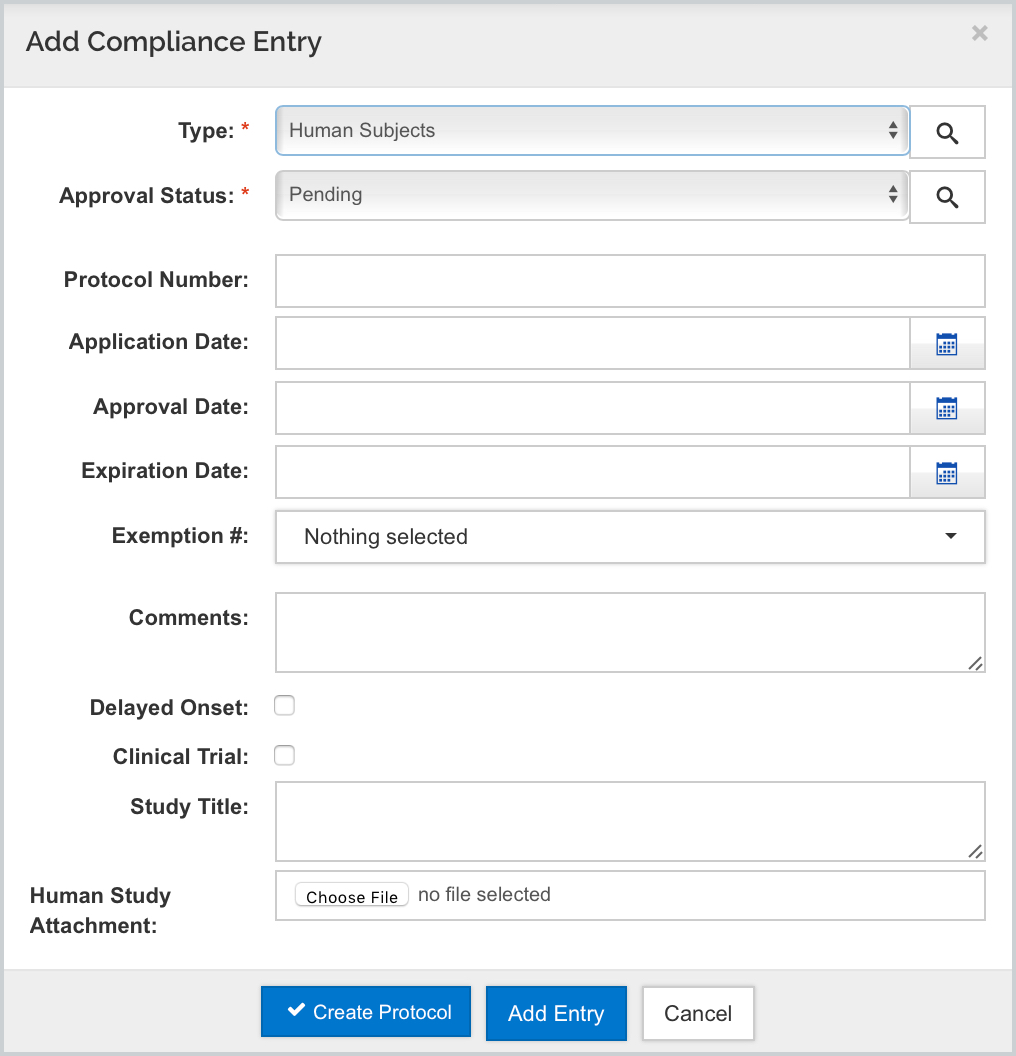
Kuali will automatically check the appropriate boxes in items 1 and 1a, and input the FWA code for the Human Subject Assurance Number which is housed in your Organization record. Be aware, that you should enter the full assurance number n the Organization record with the leading FWA at the beginning since that will get stripped when mapping to the form (i.e. FWA00000000 will map as 00000000).
The Approval Status is a selectable field when adding the Compliance entry and Pending will map YES for "Is the IRB Review Pending' - all others map NO:
- Pending - then all required information has been entered (No date or further info required)
- Submitted - enter the date of the Regulatory Review in the Application Date field.
- Approved - then a protocol number must be entered in the Protocol No. field and a date entered into the Approval Date field
- Exempt, the exempt code must be entered in the Comments field. Valid exemption codes are: E1, E2, E3, E4, E5, E6, E7, and E8 (multiple selections are allowed).
NOTE: In old/legacy IRB protocols if you have the irb.protocol.development.proposal.linking.enabled set to Y then the linked protocol information will map to the form.
2. Vertebrate Animals
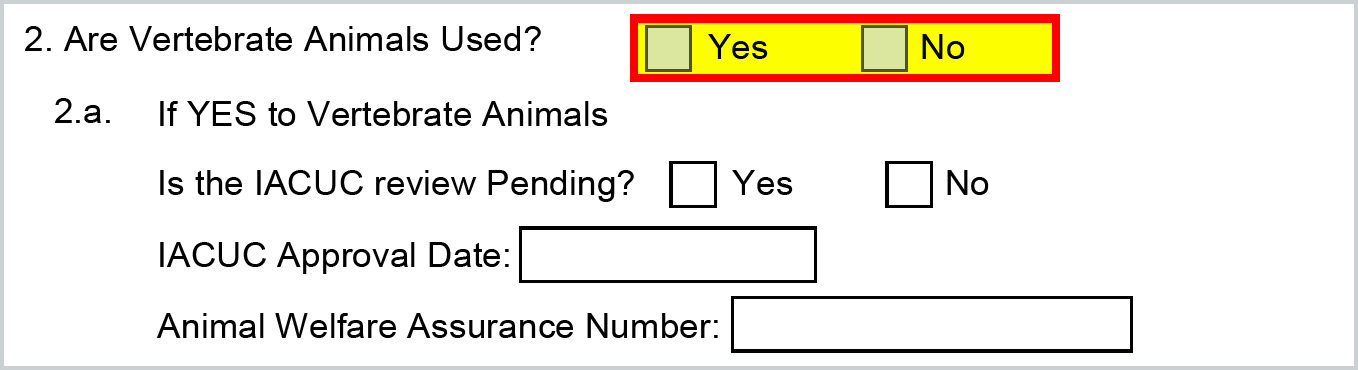
The Animal Subjects information will map based on the Special Review information you have entered in the Compliance tab of the proposal in Kuali Research. If nothing is added for Animal Subjects in Compliance then this will be marked NO. If a Special Review of Animal Subject has been added in Compliance it will mark as YES.
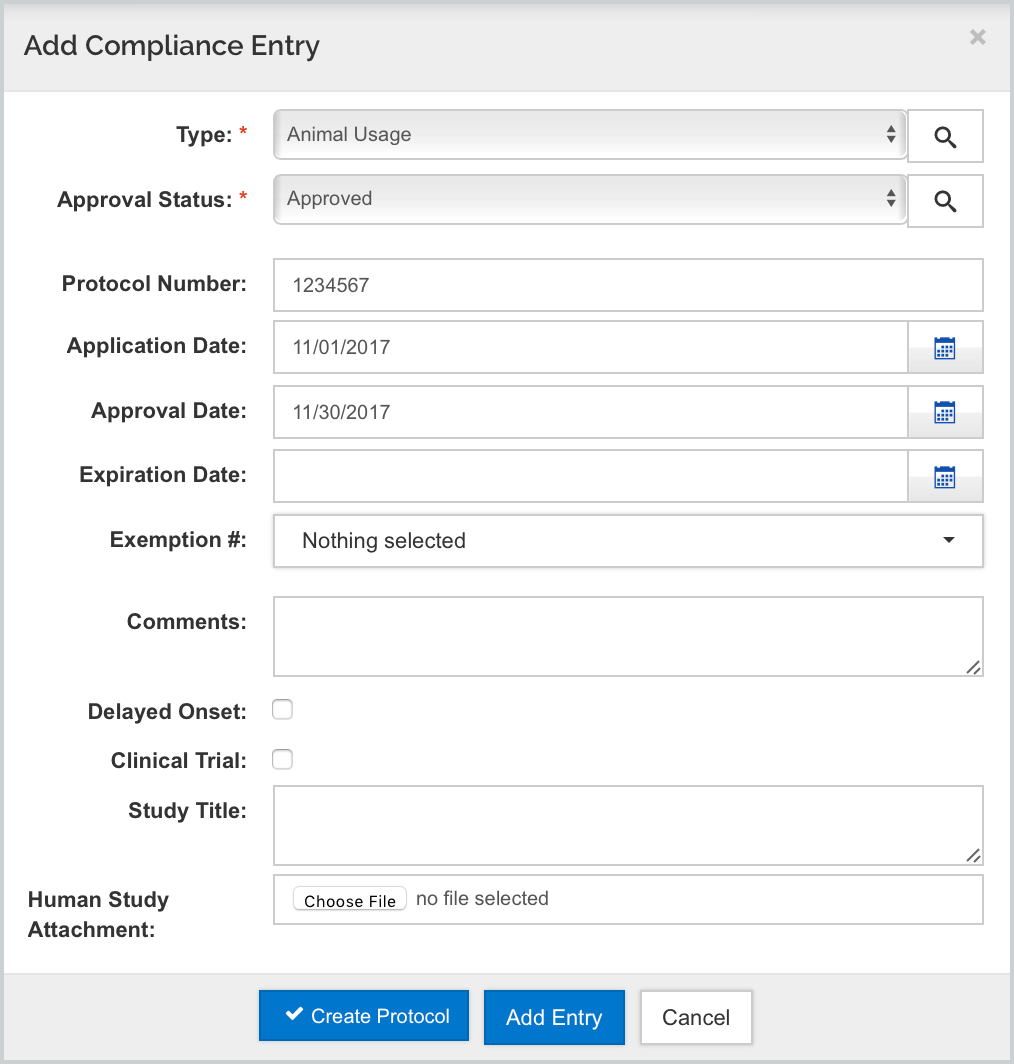
Kuali will automatically check the appropriate boxes in items 2 and 2a, and input the Animal Welfare Assurance code for the Human Subject Assurance Number which is housed in your Organization record.
The Approval Status is a selectable field when adding the Compliance entry and Pending will map YES for "Is the IACUC Review Pending' - all others map NO:
- Pending - then all required information has been entered (No date or further info required)
- Submitted - enter the date of the Regulatory Review in the Application Date field.
- Approved - then a protocol number must be entered in the Protocol No. field and a date entered into the Approval Date field
NOTE: In old/legacy IACUC protocols if you have the iacuc.protocol.proposal.development.linking.enabled set to Y then the linked protocol information will map to the form.
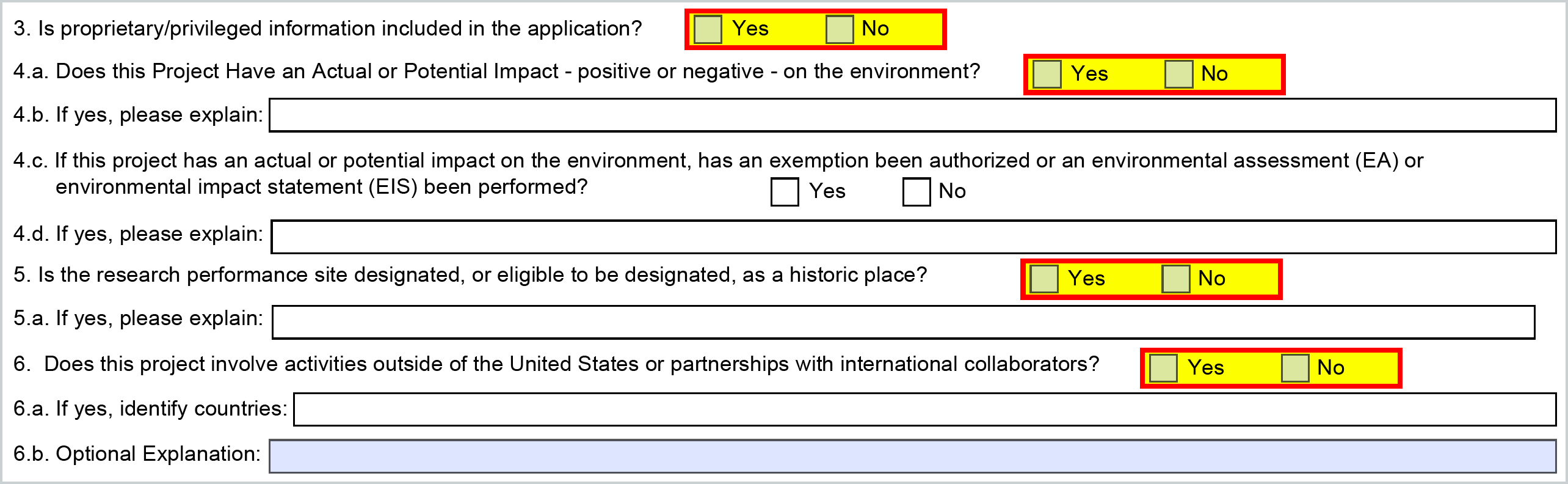
3. Questions (Items 3-6)
The below questions come from the Grants.gov S2S Questionnaire that can be completed on the Questionnaire tab of the proposal in Kuali Research.
| Form Field | Kuali Tab | Kuali Question ID | Instructions |
| 3. Is proprietary/privileged information included in the application? |
Questionnaire |
122 |
Select Yes or No. If you have marked your application texts to indicate proprietary information, check the “Yes”, otherwise, check the "No" box. Review your sponsor-specific proposal submission publication to conform to their required markings. |
| 4.a. Does this Project Have an Actual or Potential Impact - positive or negative - on the environment? | Questionnaire | 123 |
Select Yes or No. If you answered yes to 4a, additional questions will follow. |
| 4.b. If yes, please explain: | Questionnaire | 107 |
Provide a brief explanation of the actual or potential impact on the environment. (form limit 55 characters) |
| 4.c. If this project has an actual or potential impact on the environment, has an exemption been authorized or an environmental assessment (EA) or environmental impact statement (EIS) been performed? | Questionnaire | 124 |
Select Yes or No. Answer Yes to indicate an exemption been authorized or an environmental assessment (EA) or environmental impact statement (EIS) been performed. If Yes, an explanation is required in the next question. Otherwise, answer No. |
| 4.d. If yes, please explain: | Questionnaire | 107 |
Provide a brief explanation of the actual or potential impact on the environment (form limit 55 characters). If the sponsor allows, a proposal narrative type “Other” may be appropriate for a detailed explanation. |
| 5. Is the research performance site designated, or eligible to be designated, as a historic place? | Questionnaire | 125 |
Select Yes or No. A Yes answer will require an explanation. |
|
5.a. If yes, please explain: |
Questionnaire | 107 |
Provide a brief explanation for the research performance site designated or eligible to be designated as a historic place (form limit 55 characters). |
|
6. Does this project involve activities outside of the United States or partnerships with international collaborators? |
Questionnaire | 126 |
Select Yes or No. |
| 6.a. If yes, identify countries: | Questionnaire | 127 |
Enter the country(s) in the text field provided (form limit 55 characters). |
| 6.b. Optional Explanation: | Questionnaire | 107 |
Provide a brief explanation of the involvement with outside entities (form limit 55 characters). If the sponsor permits, additional explanation can be provided with a narrative file “Other”. |
Attachments (Items 7-12)
Once a Grants.gov opportunity containing the RR Other Project Info form is attached and marked as included in your federally-sponsored proposal you can follow the following instructions to populate/map the necessary attachments.
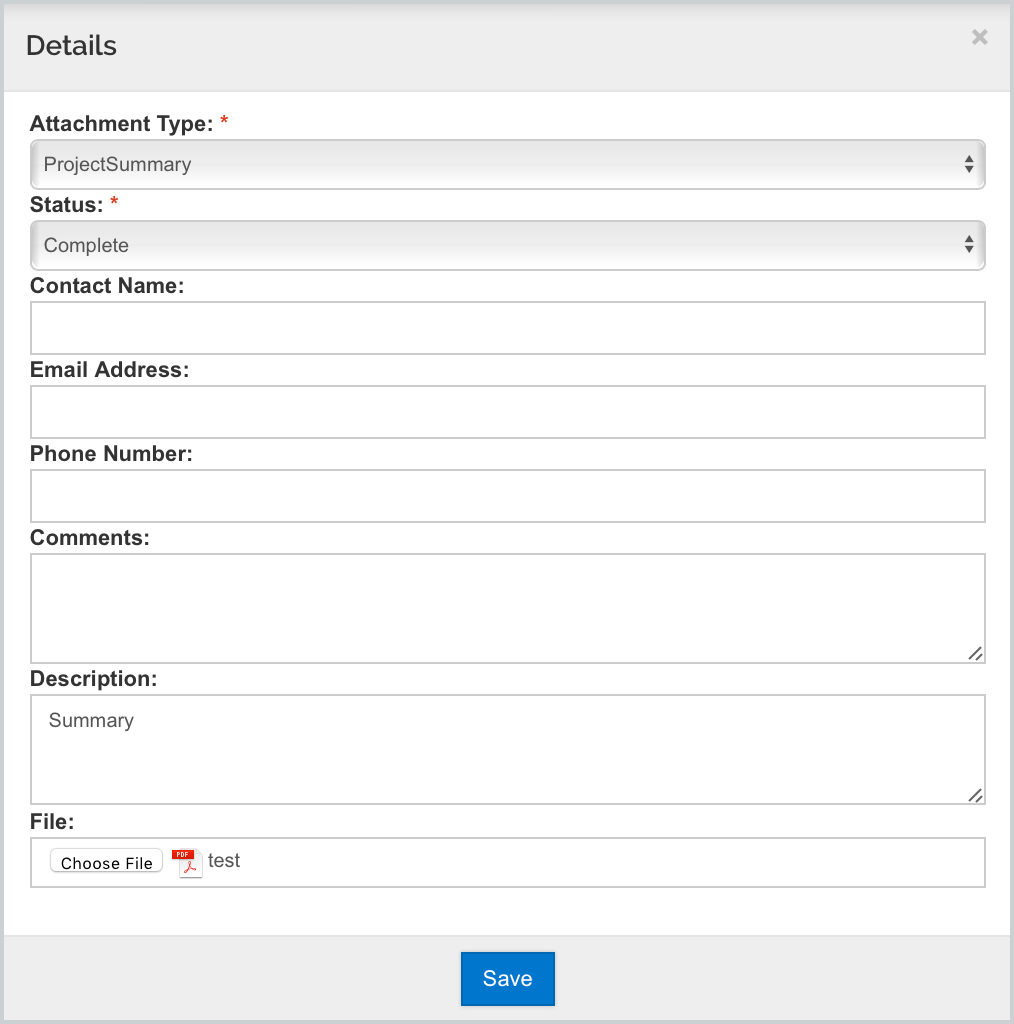
- Navigate to the Kuali Research Attachments tab -> Proposal panel
- Select the +Add button to create a new attachment
- Select the desired Attachment Type associated with this form
- For example, select 'ProjectSummary'
- Select Status of 'Complete'
- Enter a Description (optional if attachment type doesn't allow multiple)
- Upload the desired Attachment
- Click 'Save'
Repeat the above steps for the additional attachments you need to add to this form and please refer to your program announcement for instructions on the files required for the application. The below chart outlines the Attachment Types that map to the associated section in the form.
| Form Section | Kuali Attachment Type |
Kuali Narrative Type ID |
| 7. Project Summary/Abstract | ProjectSummary | 5 |
|
8. Project Narrative |
Narrative | 1 |
|
9. Bibliography & References Cited |
Bibliography | 4 |
|
10. Facilities & Other Resources |
Facilities | 2 |
|
11. Equipment |
Equipment | 3 |
|
12. Other Attachments |
Other | 8 |
Print/Submit
- After successfully uploading the attachment(s) go to the S2S Opportunity Search tab -> Forms panel
- Check the RR_OtherProjectInfo form in the select column and click the 'Create PDF' button
- Also, if this form needs to be included in the submission remember to check the 'Include' checkbox prior to final submission to Grants.gov.
- Upon Print and/or Submission the form will populate like described above with the referenced attachments appended behind the form.
Current Version
- RR_OtherProjectInfo_1_4-V1.4
Past Version(s)
- RR_OtherProjectInfo_1_3-V1.3
- RR_OtherProjectInfo_1_2-V1.2
- RR_OtherProjectInfo-V1.1
- RR_OtherProjectInfo-V1.0
Related Maintenance Table(s)
- Narrative Types
- Organization
- Questions
- Questionnaire
- Special Review Approval Status
- Special Review Type
- Special Review Usage
Related Parameter(s)
- N/A

Comments
0 comments
Article is closed for comments.