PHS Human Subjects And Clinical Trials Information Form
The Grants.gov PHS_HumanSubjectsAndClinicalTrialsInfo form can transmit the necessary information and attachments required for your application in relation to human subjects. You should check your program announcement for instructions on how to complete the form and which attachments should be included (and the formatted associated with the attachments). Information for this form is populated from the Compliance section in Kuali Research - specifically Human Subjects special review type. Also, the general Grants.gov questionnaire collects the necessary Human Specimens information. The associated attachments can be added in the Attachments and Compliance tab. Below outlines how to add the necessary attachments and other compliance information in Kuali Research to map to the given fields in the form.
Instructions
Once a Grants.gov opportunity containing the PHS_HumanSubjectsAndClinicalTrialsInfo form is attached and marked as included in your federally-sponsored proposal you can upload the necessary attachments, fill out the questionnaire question, and complete the Compliance panel to populate/map the necessary information for each section of the form.
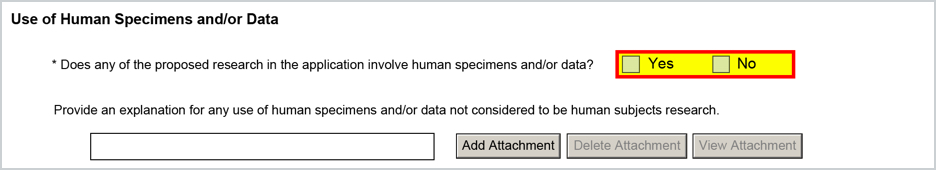
For the question 'Does the proposed research involve human specimens and/or data?' - this will will map from the S2S Questionnaire question 'Does the proposed research involve human specimens and/or data?' (Question ID: -10130). Please note, regardless of response this will only map to the form if you have not added a Human Subjects Compliance entry so the Are Human Subjects Involved response is No. It will not map if response is Yes, since human specimens question is no longer relevant.
The associated required narrative (if Human Specimen response is Yes) can be uploaded in the Attachments tab of the proposal using the following Narrative Type:
- PHS_HumanSubjectsAndCT_InvolveHumanSpecExp (Narrative Type ID: -3)
Once added the attachment will be appended to the form if this response is Yes to Human Specimans and the Human Subjects Involved flag is No.

If a Compliance entry of Human Subjects is added the answer will map as 'Yes' and pull the Exempt information if completed in the Compliance entry. If there are no human subjects for a project and nothing is added in the Compliance section of the proposal for human subjects this form will default response to 'No' for all questions and no further action is required.
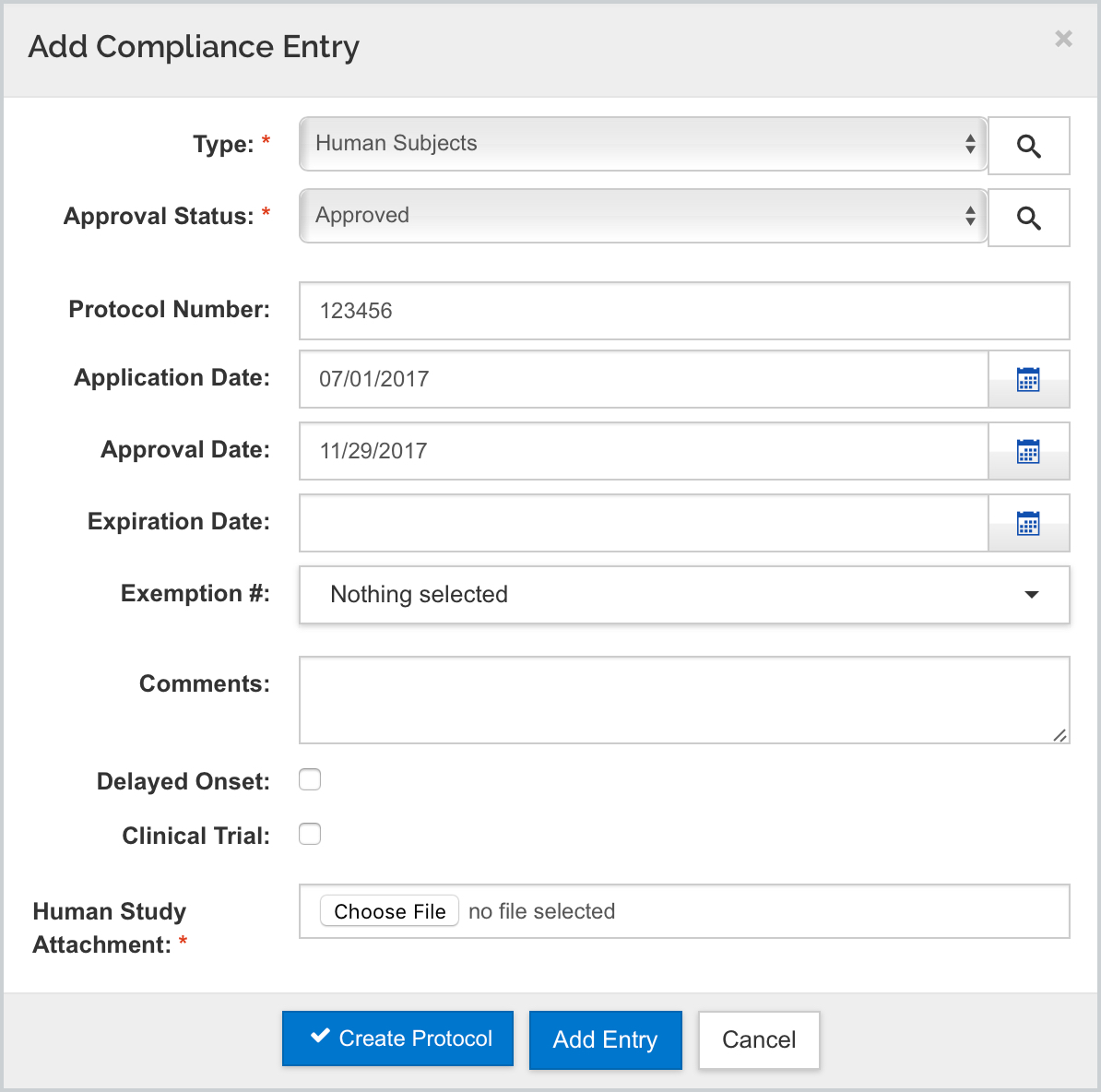
Within the Compliance tab of the proposal you can add the Special Review Type of Human Subjects. The following new fields have been added to this specific compliance entry to accommodate the PHS Human Subjects and Clinical Trials Information form:
- Delayed Onset
- Clinical Trial
- Human Study Attachment
These elements only appear for the Human Subjects type and are only used to populate this GG form. Also, these attributes do not carry forward to IP or Award Special Review section.
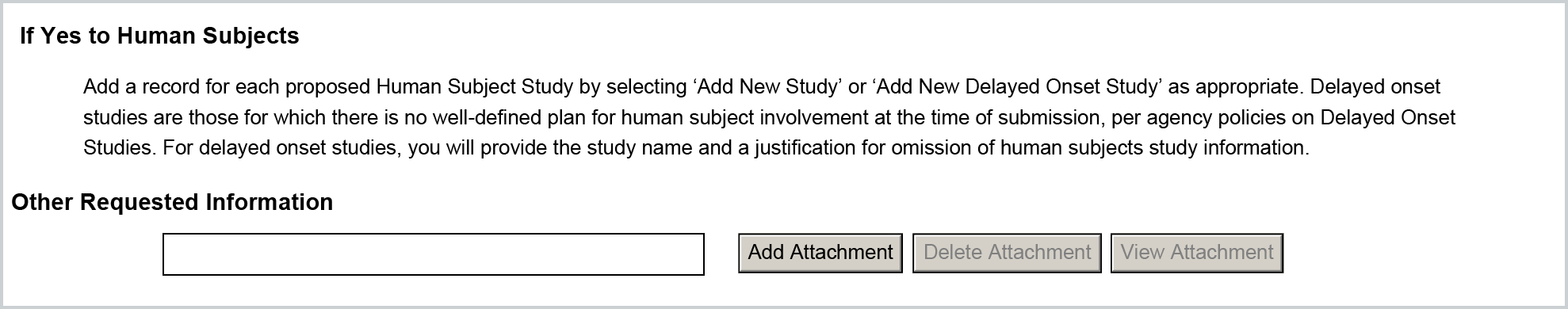
For the Other Requested Information attachment this will populate from the following narrative type uploaded in the Attachments tab of the proposal:
- PHS_HumanSubjectsAndCT_OtherRequestedInfo (Narrative Type ID: -4)
If added, the attachment will be appended to the form (NOTE: this attachment will not map if Human Subjects is No).
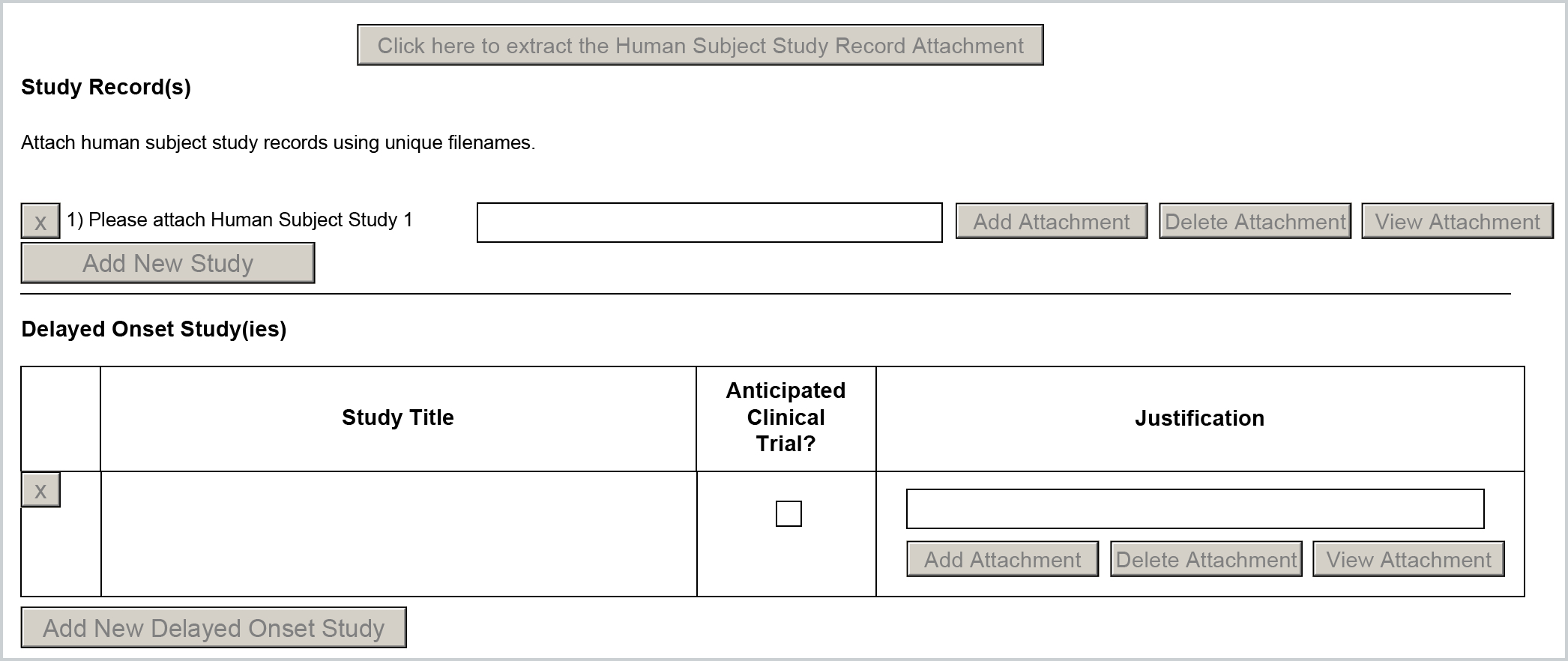
Both the Study Record(s) and Delayed Onset Study(ies) are pulled from the Compliance entries for human subjects added in the Compliance tab of the Kuali Research proposal. If multiple Human Subject entries are included it will map to the associated sections based on the selected criteria in the Compliance entry.
Study Record(s)
These entries require an extracted Human Subject Study Record Attachment form to be completed and then uploaded in the Human Study attachment field of the Compliance entry. You can extract the form from the live application by clicking on the Human Subjects Clinical Trial form in the Forms panel of the S2S Opportunity tab - be aware that the form versions must match for the form to recognize the attachment. Once added the associated form will be included in the form and appended to the PHS Human Subjects and Clinical Trial Information form.
If you have multiple Study Records to add you will need to add a separate Human Subjects entry for each in the Compliance tab of the proposal and upload an individual extracted Human Subject Study Record attachment for each.
Delayed Onset Study(ies)
If Delayed Onset you will need to check the 'Delayed Onset' checkbox in the Human Subjects Compliance entry in the Kuali Research proposal. You can also indicate whether it's a clinical trial via the the 'Clinical Trial' checkbox just below - which becomes available for entry once the 'Delayed Onset' is checked in the Compliance entry.
To include a Justification attachment you can upload a normal PDF in the Human Study attachment field of the Compliance entry. This justification will then be appended to the end of the form. Please be aware, if you have checked 'Delayed Onset' NIH eCommons will expect a flat PDF and not a fillable Human Studies PDF attachment. If a non-flat PDF is included when this is selected it will throw a eCommons validation error.
If you have multiple Delayed Onset Study(ies) to add you will need to add a separate Human Subjects entry for each in the Compliance tab of the proposal and mark the appropriate checkboxes and include separate justifications for each.
Print/Submit
- After successfully uploading the attachment(s) and compliance entrie(s) go to the S2S Opportunity Search tab -> Forms panel
- Check the PHS_HumanSubjectsAndClinicalTrialsInfo form in the select column and click the 'Create PDF' button
- Also, if this form needs to be included in the submission remember to check the 'Include' checkbox prior to final submission to Grants.gov.
- Upon Print and/or Submission the form will populate like described above with the referenced attachments appended behind the form.
KRMS Validation Options
NIH does not provide robust data validation via the XSD forms any longer. Kuali developed the NIH Validation service connection and this Service DOES provide additional warnings and errors.
However, if an institution does not use the NIH Validation Service, they will need to build custom KRMS rules around the new Compliance fields to ensure the new HSCT form populates in a way that is acceptable for NIH. To assist schools who have not moved to the new feature for pre-submission validations with NIH we build in KRMS functionality around this form.
A new KRMS function has been added for this called "humanSubjectsSpecialReviewContainsPropertyValue" (or "Is there a human subjects special review with matching property value(,)" in the Agenda UI) that takes 2 parameters -- the property you want to check and the value you want to check it against. The function returns true if there is at least one Human Subjects special review entry with a matching property value and false otherwise. You can also compare properties to "null" or "empty" to check if a field is empty or something doesn't exist (e.g. an attachment ID).
The following special review properties are supported:
| Field in the UI | Property Name |
|---|---|
| Delayed Onset | specialReviewAttachment.isAttachmentDelayedOnset |
| Study Title | specialReviewAttachment.studyTitle |
| Clinical Trial | specialReviewAttachment.clinicalTrial |
| Human Subject Attachment | specialReviewAttachment.fileDataId |
| Type | specialReviewTypeCode |
| Approval Status | approvalTypeCode |
| Protocol Number | protocolNumber |
| Application Date | applicationDate |
| Approval Date | approvalDate |
| Expiration Date | expirationDate |
| Comments | comments |
Override Compliance Permission
Since the Compliance entries are critical for this form and edits may be necessary the recently added Override Compliance permission may be useful. This permission gives users (most likely OPS) the ability to override compliance entries even on enroute proposals. More information on this functionality can be found in the Override Compliance Permission article.
Current Version(s)
- PHS_HumanSubjectsAndClinicalTrialsInfo_V3.0
Past Version(s)
- PHS_HumanSubjectsAndClinicalTrialsInfo_V2.0
- PHS_HumanSubjectsAndClinicalTrialsInfo_V1.0
Related Maintenance Table(s)
- Narrative Types
- Special Review Approval Status
- Special Review Type
- Special Review Usage
Related Parameter(s)
- n/a

Comments
0 comments
Article is closed for comments.