Overview
The Compliance screen is where you relate relevant research compliance-related information and other special permissions that may be relevant to the project (e.g. proposals that include the use of human participants in research). Aggregators can select which special reviews apply. For some special review types, the aggregator will manually enter the approval status for each review as well as any additional data related to the special review. The specific review types available for selection (beyond the Human Subjects and Animal Usage required for s2s proposals) are determined for your local business practices.
NOTE: If you utilize Kuali Protocols with the Funding Source gadget in your template you can enable the display of the linked protocol in the Compliance tab of the Proposal. If enabled and the proposal was linked in the Kuali Protocol then it will display in the Linked Protocols section of the Compliance tab. You can also click on the linked Protocol Number to open the record in the Kuali Protocols module if the user has the appropriate permissions. This section is display only to be used for reference and does not map to S2S forms or carry forward to the IP or Award records. The user will still need to manually add Compliance entries to the proposal for Grants.gov S2S and other local compliance processes. See the associated Protocols_Linking_Display_Enabled parameter article for more information on configuring this display in the proposal.
Compliance
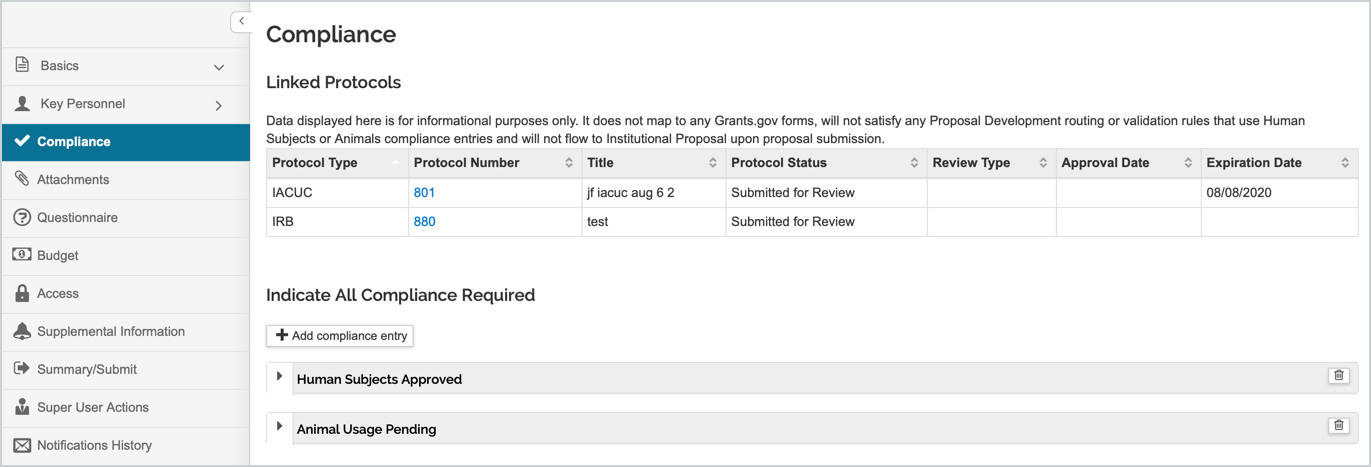
Within the Compliance tab any added Compliance Special Review entries can be added, viewed, and edited. Multiple special reviews of the same type can be added if appropriate for the application. Below outlines how to add/remove a Compliance entry and the field options that become available when entering.
Add Compliance Entry
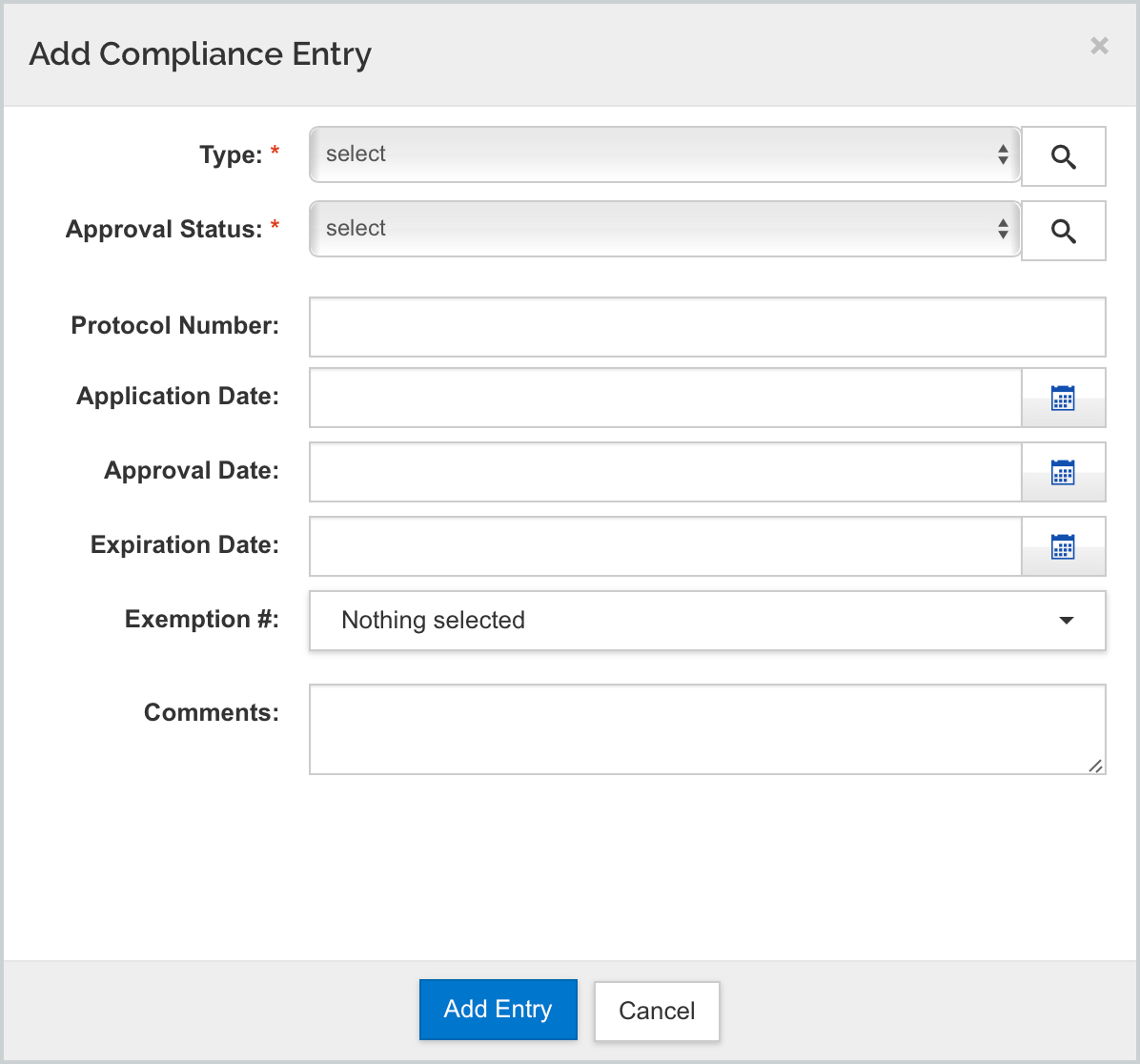
Via the +Add Compliance button you can add the desired compliance entry. Use this manual entry process if you are not using the link to the legacy IRB or IACUC module parameters:
- Click the Add Compliance Entry button
- Select a Type from the drop down list (the type selected may change the next options)
- Select an Approval Status ( the type selected may change the next options)
- Select or search for a Protocol Number, if appropriate.
- Enter or select an Application Date by clicking on the calendar tool, if appropriate.
- Enter or select an Approval date by clicking on the calendar tool, if appropriate.
- Enter or select an Expiration date by clicking on the calendar tool, if appropriate.
- Select Exemption # from the drop-down list, if appropriate.
- Enter a comment, if appropriate.
- *If maintained in your implementation, the “create protocol” button may appear. Click this button to have the system generate a new IRB or IACACU protocol document for you to complete and submit for review.
- Click Add Entry to apply this protocol/review to your proposal, otherwise, click Cancel.
NOTE: Human Subject Special Review Type has the below additional fields that are used specifically for the Human Subject Clinical Trial Information form for S2S. More information on these fields and the form mapping can be found in the S2S - 'PHS_HumanSubjectsAndClinicalTrialsInfo' Form Instructions article.
-
Delayed Onset:
-
Clinical Trial:
-
Human Study Attachment:
Delete a Compliance Entry
- On the Compliance screen, locate the item to delete
- Click the trashcan icon at the end of the compliance row.
- Click OK in the confirmation window.
- Click the Save button in the proposal footer to save the change.

Comments
0 comments
Article is closed for comments.