Overview
The Sponsor Hierarchy maintenance document is used to establish and maintain hierarchical listings of Sponsors that are made available for display and selection in various areas of the system. Kuali Research tracks sponsors at the lowest level at which your institution interacts with them. However, your institution can define in Kuali Research the relationships between individual sponsors.
Sponsor Hierarchies are a key component for reporting. For reporting, you may need to add together data for related sponsors. Use Sponsor Hierarchy to roll-up two or more sponsors into a single group.
For example, a number of individual institutes make up the National Institute of Health (NIH). Two of those institutes are the National Institute of Allergy & Infectious Disease and the National Institute on Aging. NIH, along with other sponsors like the Center for Disease Control and the Food and Drug Administration, are part of the Department of Health and Human Services (DHHS). Kuali Research tracks individual sponsored awards at the institute level, but for reporting purposes, you may need to report all NIH or all DHHS awards together.
Sponsor Hierarchies can also be used to affect Key Personnel Role functionality related to how the Proposal Award Person Role maintenance table is configured.
Sponsor Hierarchies can also be used to drive which sponsors require COI disclosure.
For example, Kuali Research can be set up by sponsor type grouping within a Sponsor Hierarchy with the below structure:
1. Sponsor Hierarchy
a. Group
i. Subgroup
Sponsor Hierarchy Rules
- You may put individual sponsors in a group and/or in a subgroup.
- A group can contain either subgroups or sponsors, but not both.
- You must have at least one group in a Sponsor Hierarchy.
- You must have at least one sponsor or subgroup in each group.
- A subgroup must contain at least one sponsor.
- You can only list a sponsor once in each Sponsor Hierarchy. In other words, you cannot put the same sponsor in two groups or subgroups in the same Sponsor Hierarchy.
- Each Sponsor Hierarchy must contain at least one group before you save it.
In the example described above, part of the sponsor hierarchy could look like this:
1. DHHS (Sponsor Hierarchy)
a. NIH (Group)
i. National Institute of Allergy & Infectious Disease (Sponsor)
ii. National Institute on Aging (Sponsor)
b. Center for Disease Control (Group)
c. Food and Drug Administration (Group)
Creating/Editing a Sponsor Hierarchy
The Sponsor Hierarchy tabbed section allows you to create a new Sponsor Hierarchy, maintain, copy an existing one, or delete one.
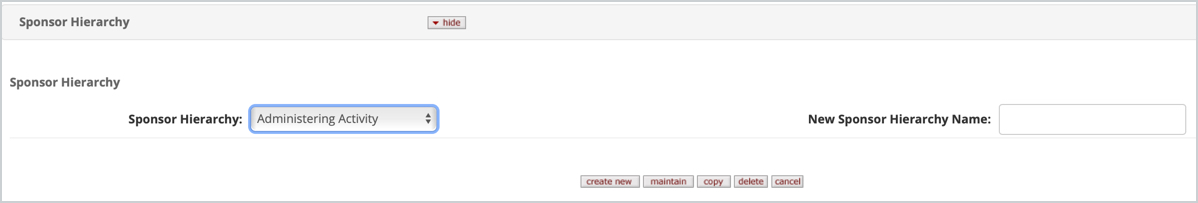
To take an action on an existing hierarchy (maintain, copy, delete) simply select the desired hierarchy from the dropdown and click the desired action button.

To create a new hierarchy you can copy an existing or you can enter a name in the New Sponsor Hierarchy Name field and then click the create new button. Once created you can then add the desired Group folder(s) to the hierarchy. Once a group is added you can then add additional groups within, edit the name of the group, change order of the folders with the up/down arrows, delete, or Add Sponsor.
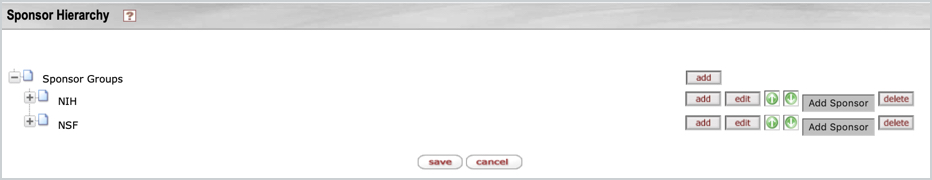
Default Sponsor Hierarchies
The sponsor hierarchies out of the box pictured above are suggestions and can be modified. But be aware that certain system functionality is driven from some of these hierarchies and how they're named and then configured in the associated parameters and Proposal Award Person Role table. Also, some S2S form mapping will use Sponsor Groups (like NIH) for logic on whether to map certain attributes like the PHS Account in the 424 coverpage rather than the EIN - if the proposal sponsor is within the Sponsor Group folder of NIH it will map the PHS Account value from the Organization record.
Related Maintenance Tables
- Proposal Award Person Role
- Sponsor
Related Parameters
- PERSON_ROLE_SPONSOR_HIERARCHIES
- ALL_SPONSOR_HIERARCHY_AS_NIH
- numberPerSponsorHierarchyGroup
- SPONSOR_HIERACHY_REQ_DIV_PROG_CODES
- SPONSOR_HIERARCHY_FOR_PRINTING
- sponsorGroupHierarchyName
- sponsorLevelHierarchy

Comments
0 comments
Article is closed for comments.