Overview
While most protocols are performed exclusively by researchers at a single institution, some compliance protocols involve multiple research institutions. In several cases a single compliance committee reviews the research being performed at each institution. Kuali Protocols provides functionality to manage External Reliance on another reviewing committee, or management tools to organize the details of other Participating Sites when your institution is serving as the primary reviewer on a multi institution study.
External Reliance
If your institution is relying on another institution to perform committee review on a Protocol you can configure a General Questionnaire gadget to identify this submission scenario so you can suppress your institution's protocol questions. The Organization Typeahead gadget can then be configured to capture the Organization providing primary review.
Once a Protocol designated as External Reliance is submitted to the compliance office for review the External Reliance Review Type can be selected. Protocols will this review type can have the Approve External Reliance action taken on them, but they cannot receive a local approval. Once this action is taken they will go into the External Reliance status.

Institutional Review of Multiple Sites
If your institution is performing review for multiple institutions taking part in a protocol you can configure your IRB or IACUC form to capture details on all Participating Sites. The first step is configuring the Reviewing IRB gadget in your protocol form. Details on this gadget can be referenced in the Compliance - Form Gadgets article. Once this gadget is included in your protocol template any researchers who indicates they are submitting a multi site protocol that requires your institution's centralized review will see the Participating Sites option appear in their protocol action bar.
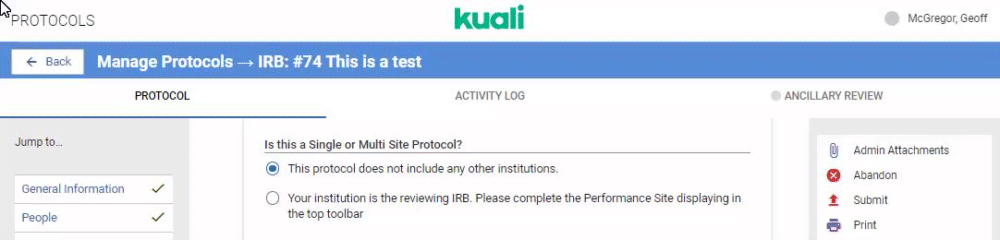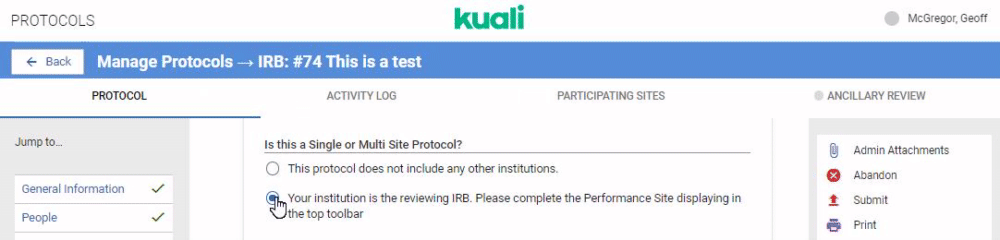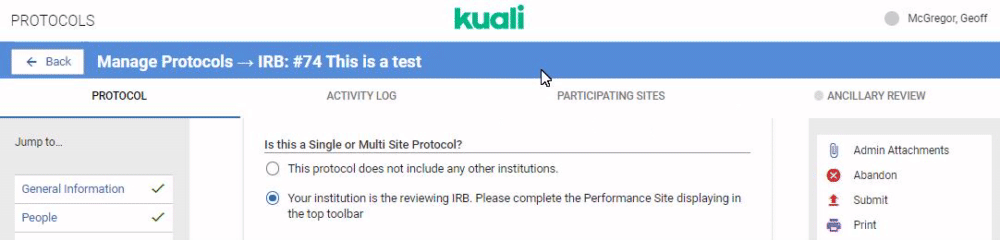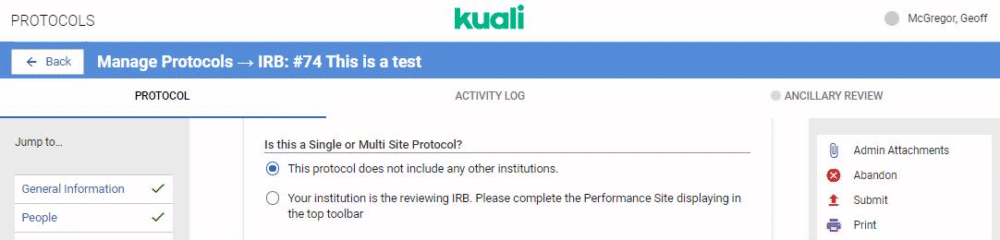
When the user clicks on the Participating Sites bar they will be given the opportunity to add Performance Sites. Performance Sites can be modified at any time to avoid requiring amendments for minor changes at a Performance Site.
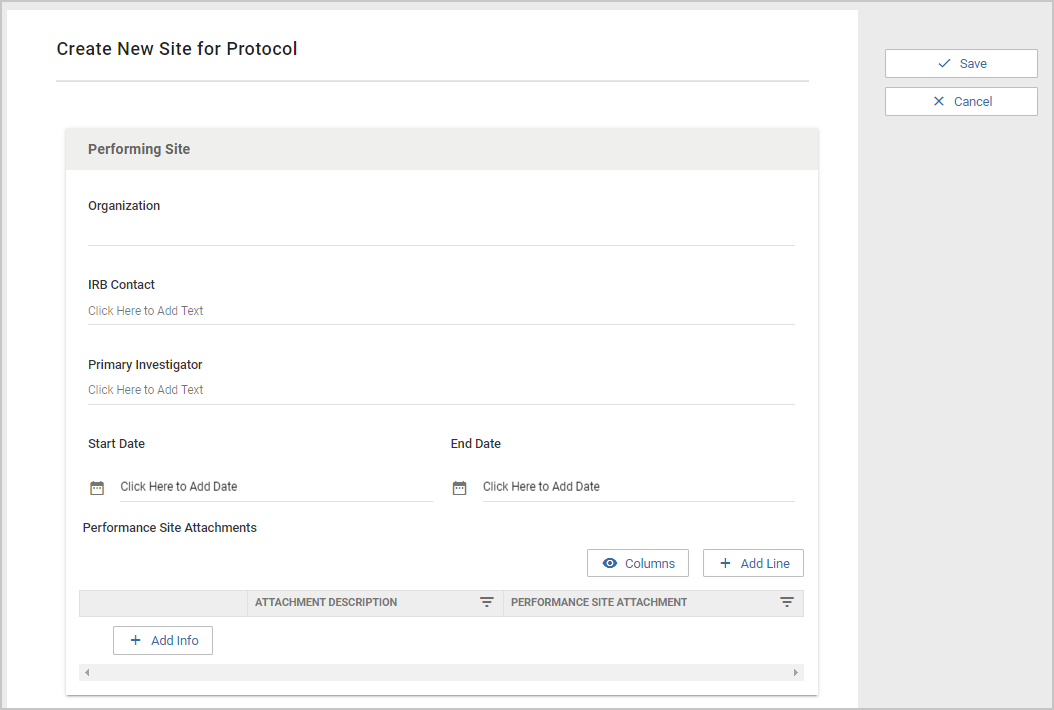
Once a user clicks on +Participating Site they will be presented with the form configured in the Participating Site Template. Each protocol type has it's own Participating Site template, so Participating Sites for IRB can have different fields than Participating Sites for IACUC. The Organization field, IRB Contact, and Primary Investigator are required by the system, but the remainder of the Participating Site fields can be customized by each implementing institution. The Organization field is a Typeahead that searches the Organization Table.
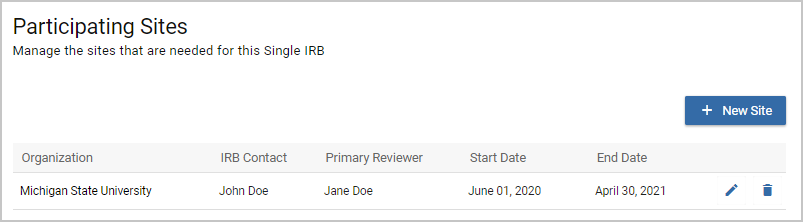
Once you've added your first Participating Site you will be able to edit or delete each site. If you edit a Performance Site you can change any of the fields except for the Organization. The Organization cannot be changed once a Participating Site is saved the first time. The Participating Sites screen will display the Organization, IRB Contact, Primary Reviewer, Start Date, and End Date listed in each Participating Site.

Comments
0 comments
Article is closed for comments.