We are happy to announce that the code to support Human Fetal Tissue* (HFT) research as outlined by NIH requirement (NOT-OD-19-137 ) has been released to production environments.
Summary of Impact
A new entry for Human Fetal Tissue Costs was added to your object code table. Kuali was able to check if SaaS schools have done any S2S submissions. In the cases that S2S was done, the object code has been included as active. So, you will see that object code available right now under your budget category of other direct costs.
The new object code (HFTC0001) for Human Fetal Tissue was automatically mapped to the MTDC rate applied in the Valid Cost Element table. If you desire other rates (i.e. inflation) to be applied to this new object code you will need to manually apply. Also, this object code was added without a Unit Number associated so it won't show up by default in the Object Code Name dropdown; a user would have to select Other Category Type, then Human Fetal Tissue in Category, and finally Human Fetal Tissue in Object Code. If you want this to appear in the Object Code Name dropdown by default you can add the top level hierarchy unit (000001) to the object code.
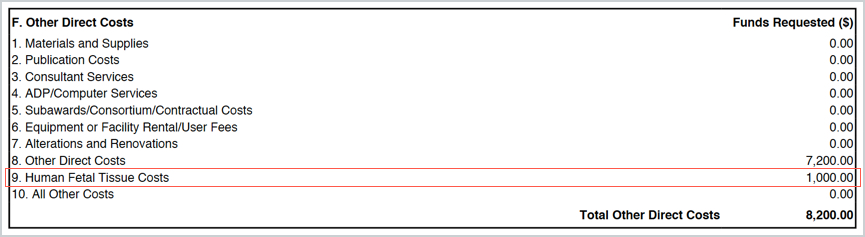
If this object code is added to your budget it will map to the RR Budget forms as a separate unique line 9 in Other Direct Costs labeled Human Fetal Tissue. If the object code is not listed in your budget, the line does not appear on the R&R budget.
Additional HFT attachment types must be specifically named and added in the Other Section of the RR Other Project Information form. Users must select the Proposal attachment type of “Other” and then add the description of either HFTSampleIRBConsentForm or HFTComplianceAssurance to each of the attachments. Both attachments MUST appear with that naming convention if HFT Research is applicable. Please Note: .pdf should not be included in the description.
Kuali can confirm that the NIH Validation Service does check to see if all attachments are added but the budget does not contain the Human Fetal Tissue Costs, or vice versa. Kuali will be defaulting the NIH Validation Service parameter to On by default.
*For the purposes of the requirements of this Notice, research involving HFT is defined as research involving the study, analysis, or use of primary HFT, cells, and derivatives, and human fetal primary cell cultures obtained from elective abortions and includes the following:
- human fetal primary or secondary cell cultures, whether derived by the investigator or obtained from a vendor.
- animal models incorporating HFT from elective abortions, including obtaining such models from a vendor.
- derivative products from elective abortion tissues or cells such as protein or nucleic acid extracts.
- any human extra-embryonic cells and tissue, such as umbilical cord tissue, cord blood, placenta, amniotic fluid, and chorionic villi, if obtained from the process of elective abortion.

Comments
0 comments
Please sign in to leave a comment.